In addition to Soberana Plus, another of the Cuban immunizers, Soberana 02, reached the Old Continent.
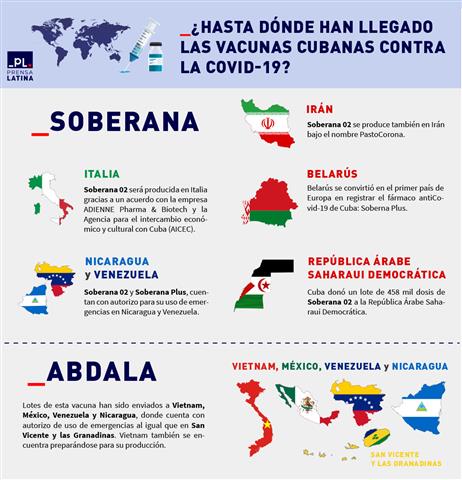
Soberana 02, designed at the Finlay Vaccine Institute (IFV) of the Caribbean island, like Soberana Plus; received the green light since April to be partially produced in Italy thanks to an agreement between said entity and the company ADIENNE Pharma & Biotech and the Agency for economic and cultural exchange with Cuba (AICEC) -both from Italy-.
The memorandum signed during the BioHabana 2022 congress will allow the island’s immunogen to be formulated, filled and packaged in the European country.
On that occasion, the general director of the IFV, Vicente Vérez, indicated that the agreement was closed thinking of a potential moment of entry of this vaccine in Europe or even in North America. “This requires alliances to share science” , he pointed.
Vérez also explained that the intention is, in a second phase, to be able to evaluate whether they could be completely produced in this Italian company Soberana 02 and other IFV vaccines.
With Italy, the threads that bind the IFV’s anti-COVID-19 products are stronger. In November 2021, some 30 people between the ages of 19 and 59 traveled from that country to Cuba to volunteer in the Soberana Plus Turin clinical study.
The objective of the trial was to evaluate the immunogen in convalescents from COVID-19 and subjects without a history of the disease, but immunized with other vaccines.
As a background to this collaborative analysis, at the “Amadeo di Savoia” Hospital in the city of Turin, sera from Cuban volunteers vaccinated with Soberana Plus were evaluated since July of last year.
The results demonstrated the ability of Soberana Plus to induce neutralizing antibodies against the alpha, beta and delta variants of the SARS-CoV-2 coronavirus, which causes the disease.
First in the world, specially designed to re-stimulate immunity previously induced by other anti-COVID-19 vaccines or by natural infection, Soberana Plus was also used in Cuba as a booster dose in the anti-COVID-19 immunization scheme, designed by the IFV of two doses of its other product Soberana 02.
The union of both demonstrated in clinical trials a 92.4 percent efficacy against symptomatic disease, according to the report of the final results of the phase III study of Soberana 02.
This was the scheme used for anti-COVID-19 immunization in children aged 2 to 18 in the country, the first campaign in the world that reached more than 1.7 million infants.
The Cuban authorities described the vaccination in pediatric ages as “total success”; Well, about 70 thousand cases were avoided, and since 2021 no child has died from COVID-19 in Cuba.
Not only in Europe are the “Sovereigns” known. Soberana 02 arrived in Iran at the beginning of 2021 when it was still a vaccine candidate, with the aim of complementing phase III of its clinical trials.
“As part of the collaboration with other countries in the development of vaccines against COVID-19, 100,000 doses of Soberana02 were sent to Iran’s Pasteur Institute, which will be used in clinical trials in that country,” announced the BioCubaFarma business group. .
By the end of June of that year, Soberana 02 received the authorization for emergency use in the Islamic Republic of Iran. The authorization was granted to the Pasteur Institute of Iran (IPI), which will market the vaccine in Iranian territory, within the framework of a collaboration agreement signed with the Finlay Vaccine Institute.
In this way, the Persian country became the first in the world to produce Cuban anti-COVID-19 vaccines.
In that nation, a center for the serial production of the PastoCorona vaccine injection was inaugurated, which became the result of the transfer, to the Pasteur Institute, of the technology of the Cuban Soberana 02 vaccine.
On this side of the world, in Nicaragua and Venezuela; Both Sovereign 02 and Sovereign Plus have authorization for emergency use.
Soberana 02 are among the vaccines approved by the Venezuelan health authorities for use in children over two years of age and in early 2022, a shipment of one million doses of Soberana Plus arrived in the Bolivarian country.
The health authorities detailed that they will use this last drug to provide protection against reinfection by the Omicron variant.
For its part, the Nicaraguan Regulatory Authority indicated since October 2021 that Soberana 02 is offered as a safe access therapeutic tool to reduce the transmissibility of Covid-19; especially in the pediatric population from two to 17 years of age.
Soberana 02 also reached the arms of children in the Saharawi Arab Democratic Republic. In February, Cuba donated a batch of 458,000 doses of that vaccine to that country for pediatric use.
Abdala also protects outside of Cuba
Although the immunizers of the Sovereign line are already known in several nations; The same thing happens with Abdala, a vaccine designed at the Cuban Center for Genetic Engineering and Biotechnology and the first in this nation and in Latin America to be conceived against the SARS-Cov-2 virus.
Millions of doses of this immunizer have arrived in Vietnam, Mexico, Venezuela and Nicaragua. When applied in a scheme of three doses every 14 days, it demonstrated an efficacy of 92.28 percent against symptomatic illness. Furthermore, it showed 100 percent efficacy in preventing severe systemic illness and death from COVID-19.
In September 2021, at the request of the Center for Research and Production of Vaccines and Medical Biologicals, the Vietnamese Ministry of Health approved the importation and use of the Abdala vaccine in this Indochinese nation.
In that same month, the first batch of the Cuban vaccine arrived in that country, made up of 900,000 doses purchased by Vietnam and another 150,000 donated by the Caribbean nation.
During the reception of that first shipment, the Vice Minister of Foreign Affairs Dang Hoang Giang thanked Cuba for the doses, of a total of 10 million agreed with the largest of the Antilles and from where the technology to produce the vaccine in Cuba will also be transferred to Vietnam. this country.
In Nicaragua, Abdala has been approved for use in emergencies since October 2021; while Venezuela signed a supply contract for 12 million units of the immunogen with the Caribbean island that same year.
At the end of 2021, the Federal Commission for the Protection against Sanitary Risks (Cofepris) of the Government of Mexico ruled in the authorization for emergency use of Abdala.
As a reference national regulatory authority, qualified by the Pan American Health Organization; Cofepris decisions are recognized by several countries in the region, so the approved vaccines are likely to be used in other nations.
Being a member of the International Conference on Harmonization of Technical Requirements for the Registration of Pharmaceutical Products for Human Use, all decisions of this authority are made based on the technical-scientific evidence presented.
In December of the same year, the Ministry of Health of Saint Vincent and the Grenadines announced on its Facebook social network account that Abdala was available at vaccination sites in that country.
With this announcement, that nation became the first in the Caribbean Community (Caricom) to authorize one of the Cuban vaccines against Covid-19.
And the WHO certification?
Despite all the evidence presented above, many skeptics will say that Cuban vaccines still lack recognition from the World Health Organization (WHO); and they are not without reason; just clarify that; Although the issue has lasted longer than expected, each country can authorize the use of a vaccine whether or not it is endorsed by that international entity.
However, this does not mean that Cuba is stopped in the process.
This was recently confirmed by the Director General of the WHO, Tedros Adhanom, who assured last May that the agency is following up on the certification process for the anti-Covid-19 vaccines created and developed in Cuba.
Since March, the Group of Biotechnological and Pharmaceutical Industries of the Caribbean nation (BioCubaFarma) detailed on its Twitter account that the WHO had been informed about the completed document to be reviewed by experts and initiate the process of international recognition of the Abdala vaccine. .
The entity pointed out in that same social network that the Center for Genetic Engineering and Biotechnology (CIGB) in charge of this immunizer began the formal exchange with the health entity in that month.
In mid-February of this year, the director of BioCubaFarma, Eduardo Martínez, explained that the company was working on the text, made up of several chapters with results on clinical and preclinical research, pharmaceutical development, as well as everything associated with facilities productive, an aspect to which adaptations have been made.
Regarding the processes followed with Abdala for this evaluation, Martínez pointed out that the decision was made to change the production site to the recently inaugurated Mariel plant, located in that industrial pole, west of Havana.
In operation since November 2021, the CIGB-Mariel Technological Industrial Park is considered the most modern in Cuba and one of the most advanced in Latin America and the Caribbean with laboratories for quality control and warehouses for raw materials and final products.
It also has plants to obtain the active ingredient of vaccines and complete immunogens in liquid, powder and spray formulations.
The objective is that the representatives of the WHO also visit the productive plants of that entity where they will carry out the necessary inspection to later obtain the permit and its inclusion in the list of products recognized by the United Nations organization, Martínez stressed.
“Cuba always maintains exchanges with the WHO/PAHO representation on everything related to the prequalification of anti-Covid-19 immunizers,” he said.
And the statement is no less true, since September of last year, the representative of the Pan American and World Health Organization (PAHO/WHO) on the Caribbean island, José Moya, reported to the AP agency about a virtual meeting between specialists from Havana, Geneva and Washington to share information, coordinate documentation and establish schedules.
Cuba’s solidarity has not only filled the world in white coats; It also arrives in several countries in bulbs that can save millions of lives. The vaccines developed on this island bear the commitment, effort and capacity of scientists with more than three decades of experience in the production of their own immunogens.
(Taken from PL)
